Sau khi đã ôn luyện các skills để làm bài thi IELTS thì bài Test sau đây sẽ giúp các bạn hệ thống lại kiến thức và thử xem trình độ của bản thân như thế nào trước khi bước vào kì thi nhé. Đây là một bài Test Reading gồm 40 câu hỏi mô phỏng theo đề thi thật, các bạn hãy canh giờ đúng 60 phút để hoàn thành phần thi này và xem mình đạt được bao nhiêu điểm các bạn nhé. Chúc các bạn học thật tốt.
READING PASSAGE 1
You should spend about 20 minutes on Questions 1–13, which are based on Reading Passage 1 below.
EXTRACTION AND PURIFICATION OF DRINKING WATER
Some consumers choose to purchase bottled drinking water, rather than relying on city tap water supplies. Bottled water has typically been extracted from underground sources. If water exists under-ground, but has no natural exit points, bottling companies may construct a water table well by drilling down to extract water from an unconfined aquifer. This is done when the Earth’s natural water level
– known as a water table – is much lower than the Earth’s surface. In some cases, as with a valley or gully on a mountain, the level of the water table may be higher than the Earth’s surface, and a natural spring can emerge. Bottling companies are permitted to extract this water from a hole drilled into the underground spring, but the composition of the water must be identical to that of the naturally surfacing variety nearby.
Artesian water is drawn from a confined aquifer, a deep underground cavity of porous rock that holds water and bears pressure from a confining layer above it. This water can be accessed if companies drill a vertical channel down into the confined aquifer. Due to the pressurised nature of this aquifer, water will often rise up from within it and form a flowing artesian well, which appears as an explosive fountain at the earth’s surface. However, this only occurs when the surface is lower than the natural water table. If the surface is not lower than the natural water table, it is still possible to draw artesian water by using an extraction pump.
Some bottled water is advertised as ‘purified’, which means it has been subjected to a variety of dif-ferent cleansing processes. A common filtering procedure, known as reverse osmosis, involves the water being pressed through microscopic membranes that prevent larger contaminants from passing through. The microscopic size of these holes is such that they can even obstruct germs, but they are most effective against undesirable materials such as salt, nitrates and lime scale. One disadvantage of reverse osmosis is that a lot of unusable water is generated as a by-product of the procedure; this must be thrown away.
For treating pathogens, an impressive newer option is ultraviolet (UV) light. Powerful UV light has nat-ural antibacterial qualities, so this process simply requires water to be subjected to a sufficient strength of UV light as it passes through a treatment chamber. The light neutralises many harmful germs by removing their DNA, thereby impeding their ability to replicate. A particularly impressive quality of UV light is its ability to neutralise highly resistant viral agents such as hepatitis.
The overall effects of UV light treatment are variable, however, which leaves many municipal water treatment processes relying on chlorination. Its powerful and comprehensive antimicrobial effect not-withstanding, chlorination is also extremely inexpensive and remains the only antimicrobial treatment capable of ensuring water remains contaminant-free all the way through the pipes and to the taps of domestic homes. Many members of the public remain suspicious of water that has been treated with such a harsh chemical. Its ease of use and affordability has meant that chlorine often plays an import-ant role in making tainted water supplies safe for consumption immediately after natural disasters have occurred.
Some water also undergoes distillation. This involves water being boiled until it converts to steam, which then passes through a cooling tube and becomes water again. Toxic compounds and impurities such as heavy metal residue are left behind in this process, so the steamed water is typically cleaner than the pre-distilled version. Unfortunately, distillation equipment also removes up to fourteen types of beneficial minerals that naturally occur in water. Consequently, those who rely on distilled water may need to take mineral supplements.
In developed countries, all forms of drinking water are typically subject to stringent quality control pro-cesses, so there is little evidence to suggest importing bottled water at significant expense will be safer or healthier than regular tap water from a municipal drinking supply. Both tap water and bottled water are tested for pathogens and contaminants and, aside from isolated cases related to issues such as faulty plumbing or old pipes, tap water is harmless. Nevertheless, many purchasers of bottled water still justify their choice on the quite reasonable basis that tap water has a distinctly unpleasant aftertaste related to the chlorination process it has undergone.
Questions 1–5
Label the diagram below.
Choose NO MORE THAN THREE WORDS from the passage for each answer.
Write your answers in boxes 1–5 on your answer sheet.
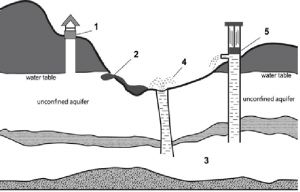
1 ……………….. (provides access to trapped water)
2 ……………….. (due to the lower land level)
3 ………………..
4 flowing artesian well (looks like ………………..)
5 ……………….. is necessary to access this water source
Questions 6–11
Classify the following statements as referring to
- reverse osmosis
- UV light treatment
- chlorination
- distillation
Write the correct letter, A, B, C or D, in boxes 6–11 on your answer sheet.
NB You may use any letter more than once.
6. It continues to protect water as it is being transported.
7. It is particularly useful during emergencies.
8. It uses a physical barrier to separate unwanted matter from water.
9. It prevents bacteria from reproducing.
10. It removes all mineral particles.
11. It produces a lot of waste water.
Questions 12 and 13
Choose TWO letters, A–E.
Write the correct letters in boxes 12 and 13 on your answer sheet. Which TWO of the following claims about water are made by the writer?
A. Bottled water is overpriced.
B.Tap water may not have a nice flavour.
C. Most people should drink bottled water.
D. Tap water is usually safe to drink.
E. Public water supplies need better maintenance.
READING PASSAGE 2
You should spend about 20 minutes on Questions 14–26, which are based on Reading Passage 2 below.
THE INTERNATIONAL STYLE
- In the early decades of the 20th century, many Western cities experienced a steep rise in demand for commercial and civic premises, due to population growth and expansion of the white-collar professions. At the same time, architects were growing discontented with the ornamental spirals and decorative features in the prevailing design ethos of art deco or art moderne. Once considered the height of sophistication, these styles were quickly becoming seen as pretentious and old-fashioned. In this confluence of movements, a new style of architecture emerged. It was simple, practical and strong; a new look for the modern city and the modern man. It was named ‘the international style’.
- Although the international style first emerged in Western Europe in the 1920s, it found its fullest expression in American architecture and was given its name in a 1932 book of the same title. The first hints of it in America can be seen on the Empire State Building in New York City, which was completed in 1931. The top of the building, with its tapered crown, is decidedly art deco, yet the uniform shaft of the lower two thirds represents a pronounced step in a new direction. Later efforts, such as the United Nations Secretariat building (1952) and the Seagram Building (1954) came to exemplify the‘true’ international style.
- The architects of the international style broke with the past by rejecting virtually all non-essential ornamentation. They created blockish, flat-roofed skyscrapers using steel, stone and glass. A typical building facade in this style has an instantly recognisable ribbon design, characterised by strips of floor-to-ceiling windows separated by strips of metal panelling. Interiors showcased open spaces and fluid movements between separate areas of the building.
- Fans of the international style of modern buildings celebrated their sleek and economical contribution to modern cityscapes. While pre-modern architecture was typically designed to display the wealth and prestige of its landlords or occupants, the international style in some ways exhibited a more egalitarian tendency. As every building and every floor looked much the same, there was little attempt to use these designs to make a statement. This focus on function and practicality reflected a desire in mid-century Western cities to ‘get on with business’ and ‘give everyone a chance’, rather than lauding the dominant and influential institutions of the day through features such as Romanesque columns.
- Detractors, however, condemned these buildings for showing little in the way of human spirit or creativity. For them, the international style represented not an ethos of equality and progress, but an obsession with profit and ‘the bottom line’ that removed spiritual and creative elements from public life and public buildings. Under the dominance of the international style, cities became places to work and do business, but not to express one’s desires or show individuality. It is perhaps telling that while banks and government departments favoured the international style, arts organisations rarely opted for its austerity.
- By the mid-1970s, the international style was ubiquitous across key urban centres, dominating skylines to such an extent that many travellers complained they could get off a plane and not know where they were. By their nature, buildings in this style demanded very little of architects in the way of imagination, and a younger generation of designers was yearning to express their ideas and experiment in novel and unexpected ways. The outcome was a shift toward postmodernism, which celebrated much of what the international style had dismissed: decoration, style without function, and an overall sense of levity. By the turn of the 1980s, the international style was considered outdated and was falling rapidly out of favour.
Questions 14–19
Reading Passage 1 has six paragraphs, A–F.
Which paragraph contains the following information?
Write the correct letter, A–F, in boxes 14–19 on your answer sheet.
14. a description of how international style buildings look on the inside
15. a reference to institutions that didn’t like to use international style buildings
16. a reason why architects didn’t like the international style
17. a building which combined art deco and international features
18. types of materials commonly used in international style buildings
19. an architectural feature previously associated with prominent organisations
Questions 20–24
Complete the sentences below.
Choose NO MORE THAN THREE WORDS from the passage for each answer.
Write your answers in boxes 20–24 on your answer sheet.
20. The development of the international style was prompted by an increased need for……………….. buildings
21. Designers used hardly any ……………….. on international style buildings.
22. International style buildings are easily identified from the outside because of the……………….. .
23 Demonstration of ……………….. and ……………….. was often an important factor in the design of old-style buildings.
24 The similarity of international style constructions reflected the concern of architects with……………….. and……………….. .
Questions 25–26
Choose the correct letter, A, B, C, or D.
Write the correct letter in boxes 12–13 on your answer sheet.
25. Some people did not like the international style because they felt it focused too much on
A. the public sector
B. differences between people
C. new ideas
D. making money.
26. In the mid-1970s
A.the best architects were no longer using the international style.
B.there was a lot of international style architecture in major cities.
C. young architects were becoming interested in the international style.
D people visited cities specifically to see international style buildings.
READING PASSAGE 3
You should spend about 20 minutes on Questions 27–40, which are based on Reading Passage 3 below.
THE MPEMBA EFFECT
In 300 BC, the famous philosopher Aristotle wrote about a strange phenomenon that he had observed: “Many people, when they want to cool water quickly, begin by putting it in the sun.” Other philosophers over the ages noted the same result, but were unable to explain it. In 1963, a young Tanzanian student named Erasto Mpemba noticed that the ice cream he was making froze faster if the mix was placed in the freezer while warm than if it were at room temperature. He persisted in questioning why this occurred, and eventually physicist Denis Osborne began a serious investigation into what is now known as the Mpemba Effect. He and Mpemba co-authored a paper in New Scientist in 1969, which produced scientific descriptions of some of the many factors at work in freezing water.
It was initially hypothesised that the warm bowl melted itself a place in the ice on the freezer shelf, thus embedding its base in a ‘nest’ of ice, which would accelerate freezing. The hypothesis was tested by comparing the result when bowls of warm water were placed on ice and on a dry wire shelf; this demonstrated that the ice nest actually had little effect. A second suggestion was that the warmer water would be evaporating at its surface, thus reducing the volume needing to be frozen, but this idea was also shown to be insignificant. Thermometers placed in the water showed that the cooler water dropped to freezing temperature well before the warmer bowlful, and yet the latter always froze solid first. Experiments at different temperatures showed that water at 50C took longest to freeze in a conventional freezer, while water initially at 350C was quickest.
On further examination, an explanation for this paradox began to emerge. Losing heat from the water occurs at the points where it is in touch with the colder atmosphere of the freezer, namely the sides of the bowl and the water surface. A warm surface will lose heat faster than a cold one because of the contrast between the temperatures; but of course there is more heat to be lost from one bowl than the other! If the surface can be kept at a higher temperature, the higher rate of heat loss will continue. As long as the water remains liquid, the cooling portion on top will sink to the bottom of the bowl as the warmer water below rises to take its place. The early freezing that may occur on the sides and base of the container will amplify the effect.
The bowl that is more uniformly cold will have far less temperature difference so the water flow will be minimal. Another inhibiting factor for this container is that ice will also form quite quickly on the surface. This not only acts as insulation, but will virtually stop the helpful effects of the water circulating inside the bowl. Ultimately, the rate of cooling the core of this body of water becomes so slow that the other warmer one is always fully frozen first. While there are limitations to this comparison (for example, we would not see such a result if one quantity were at 10C and another at 990C) this counter-intuitive result does hold true within the 5–350C range of temperatures indicated previously.
Since this paper was published, the validity of the research findings has been questioned by a number of reviewers. They point out that the initial experimental question was not clearly defined; for example, the researchers needed to decide on exactly what constituted freezing the water. They also state that the rate at which water freezes depends on a large number of variables.
Container size is one of these; for the Mpemba Effect to be noticed, the container must be large enough to allow a free circulation of water to take place, yet small enough for the freezing areas of the side and base to be effective at extracting heat too. Secondly, research at a University in St Louis, Missouri, suggests that the Mpemba Effect may be affected by water purity, or by dissolved gas in the water. Distilled water is totally free of the particles that are common in normal drinking water
or mineral water. When suspended in water, these particles may have a small effect on the speed of cooling, especially as ice molecules tend to expel them into the surrounding water, where they become more concentrated. Just as salt dissolved in water will raise the boiling point and lower the temperature at which it freezes, the researchers found that the final portion of ordinary water needed extra cooling, below zero, before all was frozen solid.
One more factor that can distort the effect is observed if the bowls are not placed simultaneously into the same freezer. In this case, the freezer thermostat is more likely to register the presence of a hotter bowl than a colder one, and therefore the change in internal temperature causes a boost of freezing power as the motor is activated.
The Mpemba Effect is still not fully understood, and researchers continue to delve into its underlying physics. Physicists cannot reach consensus. Some suggest that supercooling1 is involved; others that the molecular bonds in the water molecules affect the rate of cooling and freezing of water. A 2013 competition to explain the phenomenon run by the Royal Society of Chemistry attracted more than 22,000 entries, with the winning one suggesting supercooling as an important factor so it seems the question and its underlying explanation continue to fascinate.
Questions 27–33
Complete the summary using the list of words, A–O, below.
Write the correct letter, A–O, in boxes 29–34 on your answer sheet.
For more than 2000 years people have wondered why raising the 27 ………………… of cold water before cooling it results in more rapid cooling. At first researchers thought that a warm container created its own icy 28………………… which made the water freeze faster, but comparisons with containers resting on a dry 29 …………………indicated that this was inaccurate. Evaporation of water proved not to be a 30 …………………. . Temperature measurements showed that,although the water in the cooler container reached 00C before the warmer one, it took longer to actu-ally solidify. The water temperature drops the most at the top and sides of the container. Provided there is a temperature 31…………………, the water will continue to circulate and to cool down. Cooler water will have less water 32…………………,and thus a slower rate of freezing. If ice forms on the top of the water, this will further slow the 33………………… of freezing, but if it forms on the bottom and the sides of the container, this will increase the rate of cooling.
| A | melt | B | element | C | process |
| D | centre | E | acceleration | F | surface |
| G | factor | H | hollow | I | matter |
| J | circulation | K | limit | L | significance |
| M | theory | N | difference | O | result |
| P | temperature |
Questions 34–39
Do the following statements agree with the information given in Reading Passage 3? In boxes 35–40 on your answer sheet, write
TRUE if the statement agrees with the information
FALSE if the statement contradicts the information
NOT GIVEN if there is no information on this
34. The Mpemba Effect cannot be seen when comparing liquids with an extreme temperature dif-ference .
35. Osborne and Mpemba’s results are still widely accepted today.
36. The size of the container does not alter the Mpemba Effect.
37. Osborne and Mpemba experimented on both pure and impure water.
38. One variable is the timing and positioning of containers in a freezer.
39. Physicists now agree that supercooling accounts for the Mpemba Effect.
Question 40
Choose the correct letter, A, B, C or D.
Write the correct letter in box 40 on your answer sheet.
40. The Mpemba Effect is best summed up as the observation that
A. ice cream freezes at different temperatures.
B. different sources of heat result in water cooling at different rates.
C. salt water freezes at a lower temperature than ordinary water.
D. warmer water can freeze faster than colder water.
Academic Reading Answer Key
Reading Passage 1, Questions 1–13
1. water table well
2. natural spring
3confined aquifer
4.(an) explosive fountain
5.(an) extraction pump
6. C
7.C
8.A
9.B
10. D
11. A
12. B in either order
13.D in either order
Reading Passage 2, Questions 14–26
14. C
15. E
16. F
17. B
18. C
19. D
20. commercial and civic
21. (non-essential) ornamentation
22. (recognisable) ribbon design
23. wealth (and) prestige/prestige (and) wealth
24. function (and) practicality
25. D
26.B
Reading Passage 3, Questions 27–40
27. P
28. H
29. F
30. G
31. N
32. J
33. C
34. TRUE
35. FALSE
36. FALSE
37. NOT GIVEN
38.TRUE
39. FALSE
40. D







